Abstract
Introduction Juvenile myelomonocytic leukemia (JMML) is a rare and aggressive myelodysplastic/myeloproliferative disorder in toddlers. Ninety-five percent of patients have mutations detected in the Ras/MAPK signaling pathway including CBL, KRAS, NF1, NRAS, RRAS, RRAS2, and PTPN11. Oncogenic fusion proteins leading to hyperactive Ras signaling and other mutations in genes encoding for proteins upstream the Ras pathway have also been described. One of these upstream proteins is LNK which is encoded by SH2B3. Loss of function mutations in SH2B3 result in abnormal proliferation of hematopoietic cells due to cytokine hypersensitivity and activation of JAK/STAT signaling. Mutations in SH2B3 have been detected in a variety of hematologic malignancies, specifically in myeloproliferative neoplasms in adults. We previously identified secondary mutations in SH2B3 in JMML. Here we report a case series of four JMML patients with initiating mutations in SH2B3. We also show that SH2B3 mutant induced pluripotent stem cell (iPSC)-derived JMML like hematopoietic progenitor cells (HPC) are sensitive to JAK inhibitors including ruxolitinib.
Patients and Methods Genomic DNA from peripheral blood, bone marrow or buccal swabs was extracted using standard protocols. DNA samples were sequenced using a custom amplicon-based targeted sequencing approach. Ficoll-purified mononuclear cells from bone marrow of JMML patients and control samples were reprogrammed by using Sendai virus expressing doxycycline-regulated OCT4, KLF4, MYC, and SOX2. Control and JMML iPSCs were differentiated to HPCs by culturing in serum-free media with sequential combinations of growth factors to support multipotent hematopoietic progenitor formation. The half-maximal inhibitory concentration (IC50) for each kinase inhibitor was determined by performing Cell Titer Glo assays (Promega).
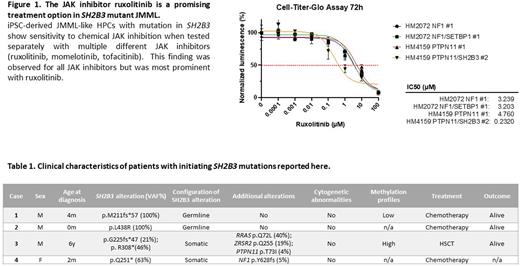
Results Clinical characteristics of patients with SH2B3 mutant JMML described in this study are highlighted in Table 1. iPSC-derived JMML-like HPCs with different mutational backgrounds were analyzed in cell proliferation assays after exposure to multiple different JAK inhibitors (ruxolitinib, momelotinib, tofacitinib). HPCs with alterations in SH2B3 were more sensitive to chemical JAK inhibition compared to HPCs not harboring mutations in SH2B3. This finding was observed for all JAK inhibitors but was most prominent with ruxolitinib (Figure 1). Patient 3 received ruxolitinib monotherapy after being refractory to 6-mercaptopurine. Treatment resulted in resolution of splenomegaly but no reduction in the variant allele frequency of SH2B3. The patient is now alive more than one year after hematopoietic stem cell transplantation (HSCT).
Discussion In a previous study of 100 patients with JMML we first identified seven patients with secondary SH2B3 mutations. Here, we report four additional patients with JMML that appear to be driven by initiating mutations in SH2B3 (Table 1). The mutations identified here result in a truncated protein (patient 1, 3 and 4) or affect the SH2 domain (patient 2). Interestingly, copy neutral loss of heterozygosity of SH2B3 in patient 1, also known as uniparental isodisomy, is a mechanism that has been observed in other genes (e.g. CBL and NF1) in JMML.
Since in vitro data showed that loss of LNK results in increased JAK/STAT signaling, we hypothesized that this cohort of patients may benefit from JAK inhibitor therapy. Our data from iPSC-derived JMML like HPCs shows, that those cells with an additional SH2B3 mutation are more sensitive to JAK inhibitors, specifically ruxolitinib, a JAK1/2 inhibitor that is already approved or under investigation in multiple clinical trials. In summary, we expand the list of initiating mutations in JMML to include SH2B3 and raise the possibility of targeting the JAK pathway in these patients.
Disclosures
French:Platelet Biogenesis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.